In case you’d like to grasp more details on Fluke Biomedical Take a look at Tools, our item industry experts are here that will help. Complete the form and anyone will provide you with a get in touch with to reply your issues.
The document discusses methods for examining new antibiotics by way of microbiological assays. It describes how the least inhibitory focus (MIC) is usually determined making use of both liquid or strong dilution methods. The liquid dilution method will involve putting together a series of examination tubes with doubling dilutions from the antibiotic staying examined and incubating which has a check microorganism.
Troubleshooting these types of issues entails very careful investigation, knowledge Evaluation, and ongoing advancement in the testing processes. Regular teaching of staff, gear upkeep, and environmental monitoring are important for reducing these difficulties and guaranteeing exact outcomes.
Sterility checks are sufficiently created in this kind of fashion they reveal the presence of contaminating microorganisms present in the examination samples useful for the experiment. It's noteworthy that sterility take a look at isn't carried out on the many products but on some agent samples of The complete great deal or batch – since It's not necessarily practicably achievable to test the many samples or products inside of a batch of solution.
This suggests which the achievable microbial contamination of the process and/or product really should be prevented website prior to it happens. Therefore, the quality methods of output and/or producing units ought to make certain aseptic process, good sterilization strategies, sterility assurance, top quality Command and assurance; as well as microbiological and Bodily parameters with the generation system should be repeatedly monitored throughout output to reduce contamination from the finished biological products.
Along with the environments where these resources are processed should often be managed inside a cleanse condition and protected against external resources of microbial contamination. Inside contamination in the clean rooms should also be prevented just as much as you can by ensuring aseptic approach at each phase with the creation.
Platforms which include Advancement Immediate® Fast Sterility detect contamination occasions earlier to aid well timed interventions, and promptly initiate root result in investigations to start mitigation.
Two standard methods are useful for microbiological assays Method A: Cylinder plate method or cup plate method. Method B: Tube assay method or titrimetric method.
But how often ought to providers conduct these assessments to strike the best stability in between performance and performance?
Killing or eliminating all kinds of microbial life (such as endospores) in a cloth or an item. Largely due to: oxidation of mobile ingredient, denature proteins, nucleic acids, RNA and loss of membrane permeability. Procedures performed in a means to prevent contamination with infectious microorganisms Used to circumvent contamination of surgical devices, health-related personnel, plus the client through surgery Sanitization: Lowering of microbial counts to forestall transmission in community placing (e.
We’ll establish the compatibility within your matrices that has a validated bioMérieux platform based upon your microbiological requirements.
Rinses enable Get well any probable microorganisms. All through bacteriostasis and fungistasis validation procedures, the antimicrobial Attributes are evaluated to make sure sterility test precision.
procedure. So, as outlined, Sterility indicators are utilised to examine the standard of sterilization and monitoring of the
Incubation: The inoculated media is incubated for at least 14 days. Normal observations are made to detect any signs of microbial expansion, and day by day information are logged for each check working day.
 Val Kilmer Then & Now!
Val Kilmer Then & Now! Karyn Parsons Then & Now!
Karyn Parsons Then & Now!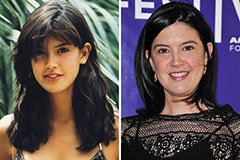 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Lynda Carter Then & Now!
Lynda Carter Then & Now! Samantha Fox Then & Now!
Samantha Fox Then & Now!